Properties Of Gases Displaying top worksheets found for – Properties Of Gases. Some of the worksheets for this concept are Properties of gases liquids solids work, Gases and their properties, Why does matter matter, Chapter practice work gases their properties an Properties of solids and liquids work, Name principles of matter, Whats the matter, Exploring the properties of gases. Gases are the least dense and most mobile of the three phases of matter.
Particles of matter in the gas phase are spaced far apart from one another and move rapidly and collide with each other often. Gases occupy much greater space than the same amount of liquid or solid. In order to standardize the literature about experiments with gases, scientists have adopted a convention whereby gas properties are often expressed at a set of conditions known as standard pressure and temperature (STP ), defined as exactly atmosphere and 273.
Topics you will need to know in order. Name(s) _____ Workshop: Gas Laws and Applications 1. Consider an ice cube made of water molecules in an absolutely empty rectangular box. The particles in a gas are well separated and do not follow any arrangement. This means they will fill the entirety of whatever space they are in and mix with particles of other gases.

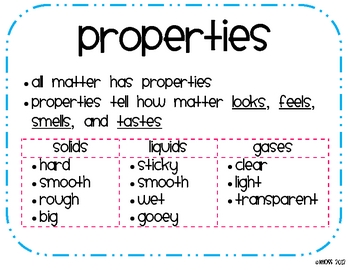
Aligned with the topic properties of the three states of matter, the chart here stimulates interest, summarizes the properties of solids, liquids and gases and assists in distinguishing between them. Article on the properties of matter and the differences between solids, liquids, and gases. Includes fill-in-the-blanks question worksheet.
Solids, liquids and gases worksheets. States of matter and changes of states worksheets and printables. These worksheets are for young learners to help with the understanding of the properties and solids, liquids and gases. The states of matter worksheets are suitable for grade or students depending on their developmental level. At low pressure (less than atmosphere) and high temperature (greater than 0°C), most gases obey the ideal gas equation: PV = nRT.
Each quantity in the equation is usually expressed in the following units: P = pressure, measured in atmospheres. V = volume, measured in liters. The three main forms of matter and the properties of solids, liquids, and gases are covered here with printables and activities that include informational texts, hands-on STEM activities and investigations, and more. Kinetic theory can help explain other properties of gases , such as their abil- ity to expand and take the shape and volume of their containers. Recall these assumptions about the particles in a gas.
All things are made of matterMatter is anything that takes up space and has mass. There are three states of Matter: Soli Liquid and Gas. Gases are compressible: when pressure is applie the volume of the gas decreases. Liquids and solids are relatively incompressible.

Once you find your worksheet , click on pop-out icon or print icon to. Density is a property of gas, and denser gases sink under less dense gases. So the distance between particles in a gas is much greater than the distance between parti-cles in a liquid or solid.
Under pressure, the particles in a gas are forced closer together, or compressed. Use this cut and stick worksheet to support KSstudents in learning the different properties of the three main states of matter. Topic 1: Air Composition and Gas Properties ELALesson Plan— Air composition and Gas Properties Description: The ELA is designed to consolidate what students have learned about the composition of air and the properties of some common gases in air, using English. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Examine kinetic energy and speed histograms for light and heavy particles.
Explore diffusion and determine how concentration, temperature, mass, and radius affect the rate of diffusion. Worksheet on Water and Air Worksheet on water and air contains various types of questions on properties of water and air. We know water takes on the taste and color of the substance dissolved in it.
Air is made up of different gases and it takes on the color of different gases.