A gas is a collection of particles in constant, rapi random motion (sometimes referred to as ‘Brownian’ motion). Gases consist of small particles (atoms or molecules) that move randomly with rapid velocities. Gas particles have little attraction for one another. Therefore, attractive forces between gas molecules can be ignored.
The distance between the particles is large compared to their size. What are the properties of gases? Liquids and solids are relatively incompressible.
The pressure of a gas The molecules of a gas , being in continuous motion, frequently strike the inner walls of their container. Molecular Interactions Key points 1. The extent of deviations from perfect behavior is summarized by the compression factor 2. The virial equation is an empirical extension of the perfect gas equation 3. The isotherms of a real gas introduce the concept of vapor pressure and critical behavior 4. Chapter The Behavior of Gases1SECTION 14. PROPERTIES OF GASES (pages 413–417) This section uses kinetic theory to explain the properties of gases.
This section also explains how gas pressure is affected by the amount of gas , its volume, and its temperature. Compressibility (pages 413–414) 1. Define pressure , and explain why a gas exerts pressure on the walls of a container. Explain the operation of a simple barometer , and why its invention revolutionized our understanding of gases.
The gas laws (b) The kinetic model of gases 1. Al-Zaytoonah University of Jordan P. Electronic books (abbreviated as e-Books or ebooks) or digital books written by Bruce E. O’Connell (Hardcover). Physical models of transport properties (viscosity, conductivity) result from the kinetic theory of gases. Methane Number during 5. Ideal Gases Experiment shows that mole of any gas , such as helium, air, hydrogen, etc at the same volume and temperature has almost the same pressure.
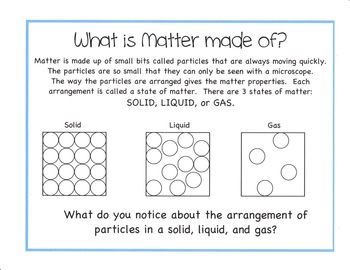
Gases will mix evenly and completely when confined to the same container. Gases are the most compressible state of matter. Gases have much lower densities than liquids and solids.
Expandability – Gases expand to completely fill their containers. Because particles are less ordered than in liquids or solids, the gas form of the same substance occupies much more space. You will be given a list of equipment and materials and some general guidelines to help you get started with each experiment. EFFECTS OF GAS ON PLANT LIFE. The four measurable physical properties described below are needed to describe the amount, state, or condition of a gas.

The ideal gas assumptions make it easier for chemists to describe the relationships between the properties of gases and allow us to calculate values for these properties. Aligned with the topic properties of the three states of matter, the chart here stimulates interest, summarizes the properties of solids, liquids and gases and assists in distinguishing between them. Properties of matter – Chart. These are often referred to as vapors. When a gas is subjected to pressure, its volume decreases.
Gases always form homogeneous mixtures with other gases. If we set the temperature and pressure for any gas to be STP, then one mole of that gas has a volume called the standard molar volume. This is another way to measure moles. The standard molar volume is 22.
For gases , the volume is proportional to the number of moles. SI Specific Heats for Ideal Gases in SI Units Table A. The computed gas thermodynamic properties in a revised graphical format which.