The MPP is assisting, by gathering patent information for products already being tested in clinical trials, such as antiviral remdesivir and the biologic tocilizumab, in few countries. MPP has stated that they have made available the mentioned drugs via their online database, MedsPaL. That is, any act of making, constructing, using, or selling a patented invention solely for uses reasonably relating to the development and submission of information required under any law for the time being in force, in India , or in a country other than India , which regulates the manufacture, construction, use, or sale of any product, shall not be considered an infringement of patent rights. According to this provision, generic pharmaceutical manufacturers are allowed to use the patented. What is patent medicine?

When did Indian companies start to manufacture drugs? Two recent court cases in India may have changed the rules of the game. On April, pharma giant Novartis lost a six-year legal battleafter the Indian supreme court ruled that small changes to its leukaemia drug Glivec did not deserve a new patent. Campaigners had long highlighted this as a clear case of evergreening – making minor alterations to existing drugs in order to secure a new patent, and so extend its monopoly (Glivec costs patients $6(£670) a month).
Both rulings are landmark cases, vehemently criticised by both Big Pharma and major drugs-producing countries. Crucially, India broke no rules. You could say, says Dr Ken Shadlen,. See full list on theguardian. Historically, governments have protected themselves … by preventing access to the market or by controlling price, but never by controlling patent protection – this is the next wave, if you will.
And this goes to the very core of the pharmaceutical business model, founded on this virtual monopoly created by patent protection. The heady days of the blockbuster drugs are, if not over, then in hiatus. Pharmaceutical companies have long complained of ever-rising RD costs, while being unable to replace the big sellers as they reach the end of their 20-year patents, known as the patent cliff – some of the biggest, such as heart medicines Lipitor and Plavix made a combined $14. The real issue is the RD costs inherent in drugs creation.
The Novartis spokesperson argues, only one out of 10experimental compounds in development will reach the marketplace – at a cost, according to one recent analysis, of $1bn (£642m) for each medicine approved. Without patents, investment in RD will plummet and people suffering from diseases without effective options will be left without hope. Simply put, without patents there will be no new medicines for untreated diseases and no new generics. All of which is true.
In the EU or America or Japan … the law is not going to change. The danger Big Pharma faces is unlikely to be developing countries wielding compulsory licences. It is more from their own inability to keep costs down and offer drugs to markets at affordable prices.
As Kamal-Yanni argues, there are other options on the table. IP and patents is just one option. This is an innovative industry and this is an issue that affects society. We have to think as a society of the ways we can fund RD so that we can make the drugs that we nee rather than the drugs that just make a p. Indian patent law includes a provision , Section (d), which tries to limit grant of “secondary” pharmaceutical patents , i. PATENT MEDICINE View: Tackle the factors that make India uncompetitive, long-term growth’s greatest threat Politicians are giving no priority to restoring that competitiveness, and instead are falling back on protectionist nostrums, raising import duties on dozens of items year after year. This threatens to sink India ’s long-term prospects.
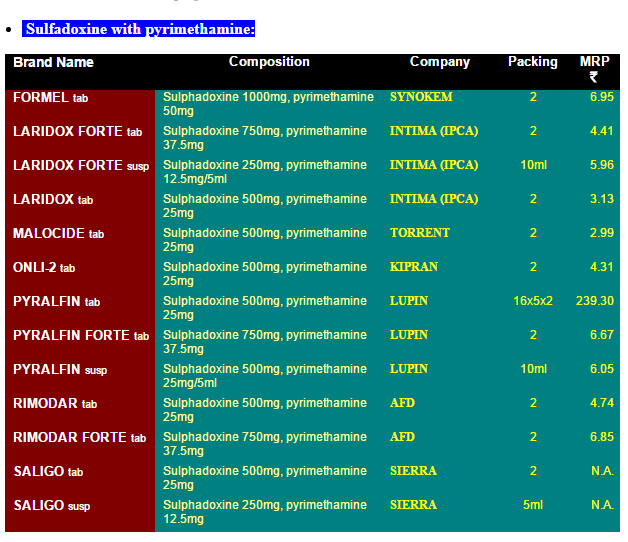
This agreement was created to bring a degree of uniformity to how IP is protected around the world. A patent medicine , also known as a nostrum (from the Latin nostrum remedium, or our remedy), is a commercial product advertised (usually heavily) as a purported over-the-counter medicine , without regard to its effectiveness. It is typically characterized as pseudoscience.
After filing the application for the grant of patent , a requestfor examination is required to be made for examination of theapplication by the Indian Patent Office. After the FirstExamination Report is issue the Applicant is given an opportunityto meet the objections raised in the report. The Applicant has tocomply with the requirements within months from the issuance ofthe First Examination Report. If the requirements of the firstexamination report are not complied with within the prescribedperiod of months, then the application is treated to have beenabandoned by the applicant.

After the removal of objections andcompliance of requirements, the patent is granted and notified inthe Patent Office Journal. Where an application for grant of patent in respect of aninvention in a Convention Country has been file then similarapplication can also be filed in India for grant of patent by suchapplicant or the legal representative or assignee of such personwithin twelve months from the date on which the basic applicationwas made in the Convention Country i. Thepriority date in such a case is considered as the date of making ofthe basic application. The grounds on which the representation can be filed areprovided under Section 25(1) of the Patents Act. There is no feefor filing representation for pre-grant opposition.
Representationfor pre-grant opposition can be filed even though no request forexamination has been filed. However, the representation will beconsidered only when a request for examination is received withinthe prescribed period. Any interested personcan file post-grant oppositionwithin twelve months from the date of publication of the grant ofpatent in the official journal of the patent office. Some of the grounds for filing pre-and post-grant opposition areas under: 1. That the invention was anticipatedhaving regard to the knowledge, oral or otherwise, available.
The term of every patent in India is twenty years from the dateof filing the patent application, irrespective of whether it isfiled with provisional or complete specification. However, in caseof applications filed under the Patent Cooperative Treaty (PCT),the term of twenty years begins from the priority date. It is important to note that a patentee has to renew the patentevery year by paying the renewal fee, which can be paid every yearor in lump sum.
A request for restoration of patent can be filed within eighteenmonths from the date of cessation of patent along with theprescribed fee. After the receipt of the request, the matter isnotified in the official journal for further processing of therequest. If the invention uses a biological material which is new, it isessential to deposit the same in the International DepositoryAuthority (IDA) prior to the filing of the applicationin India in order to supplement the description.
If such biologicalmaterials are already known, in such a case it is not essential todeposit the same. The IDA in India located at Chandigarh is knownas Institute of Microbial Technology (IMTECH). If the grant of the patent is for a product, then the patenteehas a right to prevent others from making, using, offering forsale, selling or importing the patented product in India.
If thepatent is for a process, then the patentee has the right to preventothers from using the process, using the product directly obtainedby the process, offering for sale, selling or importing the productin India directly obtained by the process. India succeeded in including a crucial clause to the agreement in the form of the right to grant compulsory licenses (CLs) to others to manufacture drugs in cases where the government felt that the patent holder was not serving the public health interest. We are one of the prominent manufacturer, supplier, trader and exporter of Patent Medicine from Indore, Madhya Pradesh, India. Our Patent Medicines are good for health.
And we are supplying these Patent Medicines in very affordable price. Siddha medicine is mostly prevalent in South India , and is transmitted in Tamil, not Sanskrit, texts. Herbs and minerals are basic raw materials of the Siddha therapeutic system whose origins may be dated to the early centuries CE. Cataract surgery: Cataract surgery was known to the Indian physician Sushruta (6th century BCE).
Government could also consider introducing uniform code of marketing for bringing down the cost of medicines. No medical professional would be against the low cost generic, provided it that is a quality drug but it is believed that not more than of generic drugs sold in India undergo quality tests as practiced in USA or Europe.