Bond Order is and it is Paramagnetic. There are two MO diagrams you need to memorize for diatoms ( N, O Ne etc). One is for the elements up to Nitrogen. This is the reasoning for the rearrangement from a more familiar diagram.
Here is the full molecular orbital diagram for N 2. Now we add the electrons, from each nitrogen atom. Note that the bottom sigma symmetry orbital is strongly bonding, the top one is strongly antibonding, and the in the middle are only weakly bonding and antibonding, respectively. Nmolecular orbital energy level diagram picture, is usually depicted by a diatomic molecules.
How to do orbital diagrams? What is molecular orbital model? The short answer is: we could not tell it using the primitive molecular orbital theory introduced in the general chemistry courses. If we build the MO diagram for N _ , it looks like this: First though, notice that the p orbitals are supposed to be degenerate. Anyways, for the electron configurations, you would use a notation like the above.
MO diagram is different for molecules with or less electrons than the one used for molecules with or more electrons. For Nthe orbitals in increasing energy are: σ1sσ∗1sσ2sσ∗2sπ2px,π2pyσ2pzπ∗2px,π∗2pyσ∗2pz because it has electrons. Jmol models of calculated wavefunctions. To view a model, click on a molecular orbital in the energy level correlation diagram shown Mouse Control of Models.
Molecular Orbitals for N2. Let me explain the molecular orbital diagram of Nusing its diagram. Visit the post for more. According to the molecular orbital theory, the general molecular orbital configuration will be, As there are electrons present in nitrogen.
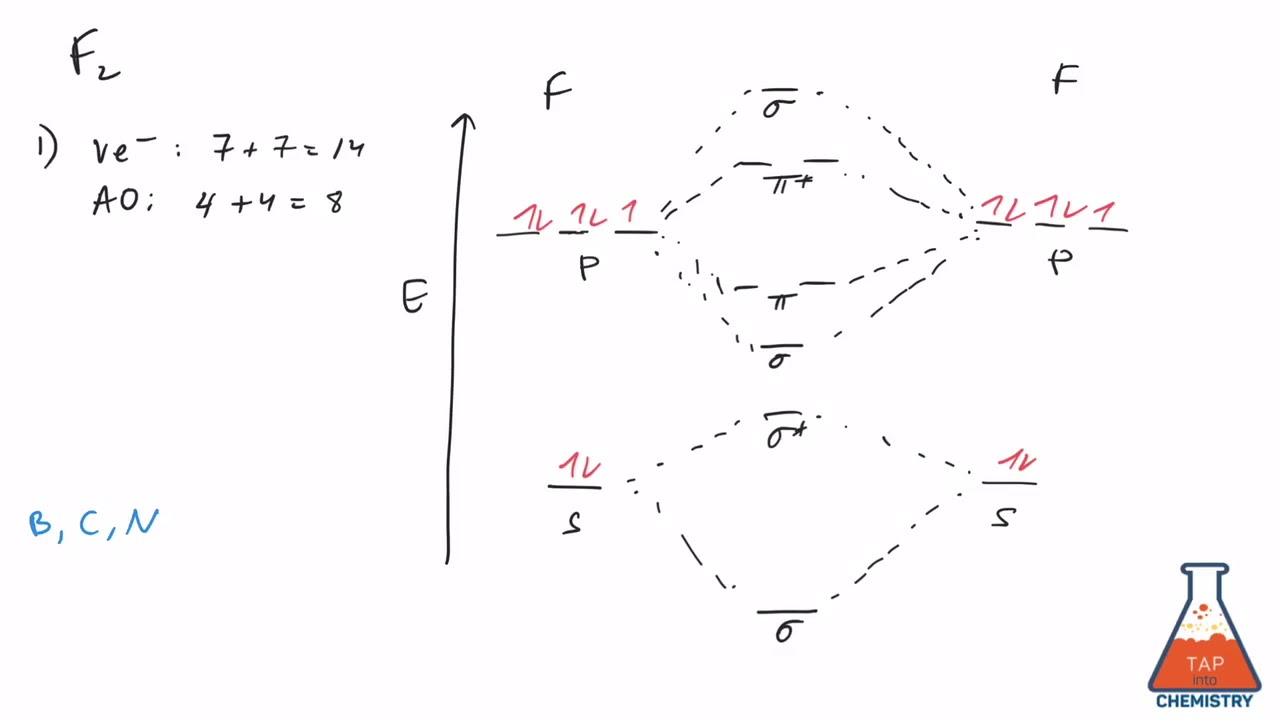
The number of electrons present in molecule = 2(7) = 14. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right.
Each horizontal line represents one orbital that can hold two electrons. Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. Photoelectron spectroscopy provides useful information on the energies of atomic orbitals.

Next we’ll see that symmetry will help us treat larger molecules in the LCAO-MO theory framework. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involve we refer to these orbitals as molecular orbitals. As the overlap between two atomic orbitals increases, the difference in energy between the resulting bonding and antibonding molecular orbitals increases. Also, does anyone know the orbital diagram (condenced) for Mn?
Mn is paramagnetic or diamagnetic and how to know that? In the n=shell you only find s orbitals , in the n=shell, you have s and p orbitals , in the n=shell, you have s, p and d orbitals and in the n=up shells you find all four types of orbitals. It is important to note here that these orbitals , shells etc. This corresponds well with the Lewis structure ( ), although the orbital approach tells us that there is one s and two p. Then we rank them in order of increasing energy.

We can ignore the 1s orbitals , because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ.