What four properties of gases can be measured? Where can you find list of all known gases? Properties of Gases : 1. It acquires the shape of the container. ADVERTISEMENTS: Reason:.
Reason: Molecules escape from an open container. A gas has no surface of its own. The molecules have the standard physical properties of mass , momentum , and energy.
And these properties are related to the macro properties of density , pressure , and temperature. List four properties of most metals? Can you list four size independent properties? The specific relationships among these four variables are the gas laws, and a gas whose behavior follows these laws exactly is called an ideal gas. The following list has substances known to be gases, but with an unknown boiling point.

Elements or compounds that are Gases at Room Temperature. Before examining the chemical and physical properties of gases , it might be useful to ask: What kinds of elements or compounds are gases at room temperature? To help answer this question, a list of some common compounds that are gases at room temperature is given in the table below.
See full list on chemed. On the other han increasing temperature and decreasing pressure allows particles to move father apart. Depending on the conditions, a substance may skip a phase, so a solid may become a gas or a gas may become a solid without experiencing the liquid phase.

In fact, they did not determine that gases constituted a state of matter until the 17th century. Upon closer study, they began observing consistent properties that defined gases. There are four differently properties of gases that can be measured and that relate to each other through various laws we will discuss later in the unit.
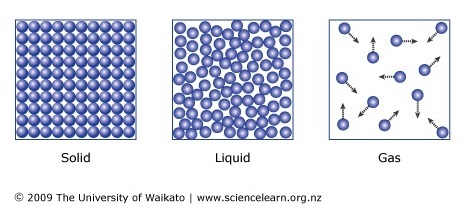
These properties are volume, temperature, amount and pressure. Each of these has a single letter commonly used to symbolize that property. All gases share common physical properties. Like liquids, gases freely flow to fill the container they are in.
But while liquids have a defined volume, gases have neither a defined volume nor shape. And unlike liquids and solids, gases are highly compressible. The Four Gas Law Variables: Volume, Temperature, Pressure, and Amount. The three-dimensional space enclosed by the container walls is called volume. When the generalized variable of volume is discusse the symbol V is used.
The motion is associated with average kinetic energy that is. Gas molecules move constantly and randomly throughout the volume they occupy 3. Utilization of the Hall-Heroult process in aluminum production in this gas. Additionally, it is used as a refrigerant.
CFis a strong greenhouse gas that contributes to climate change and has an atmospheric lifetime of 50years. Number of gas particles in the sample. Hope this helps and good luck. A pure gas may be made up of individual atoms (e.g. a noble gas or atomic gas like neon), elemental molecules made from one type of atom (e.g. oxygen ), or compound molecules made from a variety of atoms (e.g. carbon dioxide).
Define pressure, and explain why a gas exerts pressure on the walls of a container. Explain the operation of a simple barometer, and why its invention revolutionized our understanding of gases. This is the basis of the ‘ideal gas ’ approximation, of which more later. GWPs for a few selected gases are given in the table.
To interpret GWPs, consider, for example, the year GWP of for CH 4. The four measurable physical properties described below are needed to describe the amount, state, or condition of a gas. Oxygen gas will produced via a decomposition reaction of a certain substance. Name the substance that will be decomposed.