
Sample informed consent for Qualitative Research. Institutional Review Board University of Public Health, Yangon Ministry of Health and Sports Republic of the Union of Myanmar. Informed Consent Form for Qualitative Research Name the group of individuals for whom this consent is written.
Because research for a single project is often carried out with a number of different groups of individuals – for example counselors, community members, clients of services – it is. Subjects should be informed in clear, concise language about the nature of the study and the purpose for conducting the research. The total number of subjects involved and a. When this is the case, using, sharing, and re-using qualitative data may be more likely to result in human participants’ identities being disclosed. Obtaining informed consent should involve open communication between you and the individuals whom you invite to participate in your study.
Much attention has been given to the consent document readability and its comprehension. However, it is important to remember that the document is a proxy and reference for a conversation. Protecting human participants in research is extremely important, and part of that process is informed consent. One challenging aspect of this process is successful communication of risks and benefits to potential research participants.
What is informed consent? This study explored the opinions and attitudes of informed consent experts about conveying risks and benefits to inform the development of a survey about the perspectives of research nurses who are responsible for obtaining informed. It is not merely a form that is signed but is a process, in which the subject has an understanding of the research and its risks. The researcher is also interested in the factors that keep players motivated to continue with tennis.
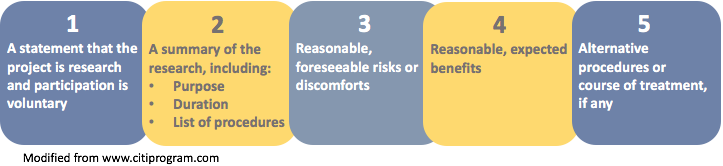
Informed consent is essential before enrolling a participant and ongoing once enrolled. The informed consent form consists of two parts: the information sheet and the consent certificate. Do not be concerned by the length of these templates.

They are long only because they contain guidance and explanations which are for you and which you will not include in the informed consent forms that you develop and provide to participants in your research. It is each researcher’s responsibility to solicit consent in a way that is appropriate for the research content and context (e.g., “group consent” in traditional societies). Two types of documentation are helpful in doing so: a project information sheet that describes your project, and an informed consent form (or verbal script) that solicits a potential participant’s consent to be included in it. It includes: Providing specific information about the study to subjects in a way that is understandable to them.
However, the conditions under which you may be required to obtain permission from those being observed varies. This template is for research interventions that use questionnaires, in-depth interviews or focus group. As a result, these patients are often very vulnerable to the researchers’ behaviour.

Abstract: This paper highlights the main issues concerned with preserving fieldwork contracts, such as informed consent agreements, as they relate to the conduct of research and the archiving of qualitative data. We pay particular attention to the techniques and efficacy of anonymisation an outline methods of gate-keeping for access to data. The paper explores issues of informed consent in qualitative social research in general but focuses specifically on research conducted with so called ‘vulnerable’ groups (to include children, older people and people with a range of physical and mental health problems) in that issues of consent are perceived as being particularly pertinent when conducting research with these groups. The consent process typically includes providing a written consent document containing the required information (i.e., elements of informed consent ) and the presentation of that information to prospective participants. I am conducting a qualitative research study on computer purchase habits.
The Research The purpose of this study is to gain insight into why college students make the switch from personal computers to Apple computers. This consent form asks you to allow the researcher to record and view the interview and to use your comments to enhance understanding of the topic. The form also asks your permission to use related observations, images or posts as data in this study. The research studies the author uses to illustrate the issues of informed consent depend on building up a relationship of trust with the participant in order to obtained consent. It is further suggested that a process consent method is a necessary requirement for person-centered research.
Again, what consequences ensue. Written (signed) consent is the standard. The form contains a list of statements which must be checked off before the document can be signed to indicate to participants full consent.
As part of the informed consent , researchers commonly communicate the methods of storing, using, and possible access to the research material. However, the question arises of whether the consent covers casual conversations about the researche the research itself or sharing particular information with other researchers, friends, or family. It also means that participants exercise their rights as autonomous persons to voluntarily accept or refuse to participate in the study.