
Using MOT the bond order of Ccomes out to be but remember in the molecules like Band Cthere is no sigma bond. The bond order of Cmolecule is 2. Answer and Explanation:. One analysis suggested instead that a quadruple bond exists, an interpretation that was disputed. Highest bond order means highest bond energy and shortest bond length. So, the highest bond order with highest bond energy and the shortest bond length is found in C2.
What is the bond order of C 22-? When the two carbon atoms. How do you calculate bond order? Is there a quadruple bond in C2? Why both the bonds in Cmolecule are pi bonds?
The electrons in the bonding orbitals are identified as being in two pi bonding orbitals. In accordance with C. Once on file with CBP, the Cbond is valid for all ports of entry. Fill from the bottom up, with electrons total. While a compound can have a bond order of zero, this value is not possible for elements.
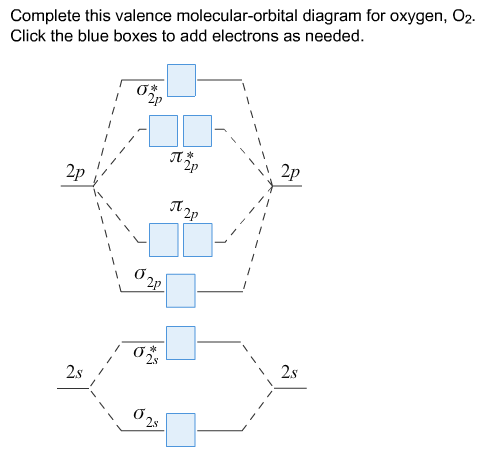
Determine bond order at a glance. In its most basic form, the bond order is the number of bonded electron pairs that hold two atoms together. A molecule of hydrogen gas (H 2) has single bond and a bond order of 1. The triple bond of CN gives it a bond order of 3. Supplementary Section III).
These bond orders correlate with the above D in (total) values we. For this, we need to do the following steps: Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Other things being equal, which they are in this case, the highest bond order has the highest.
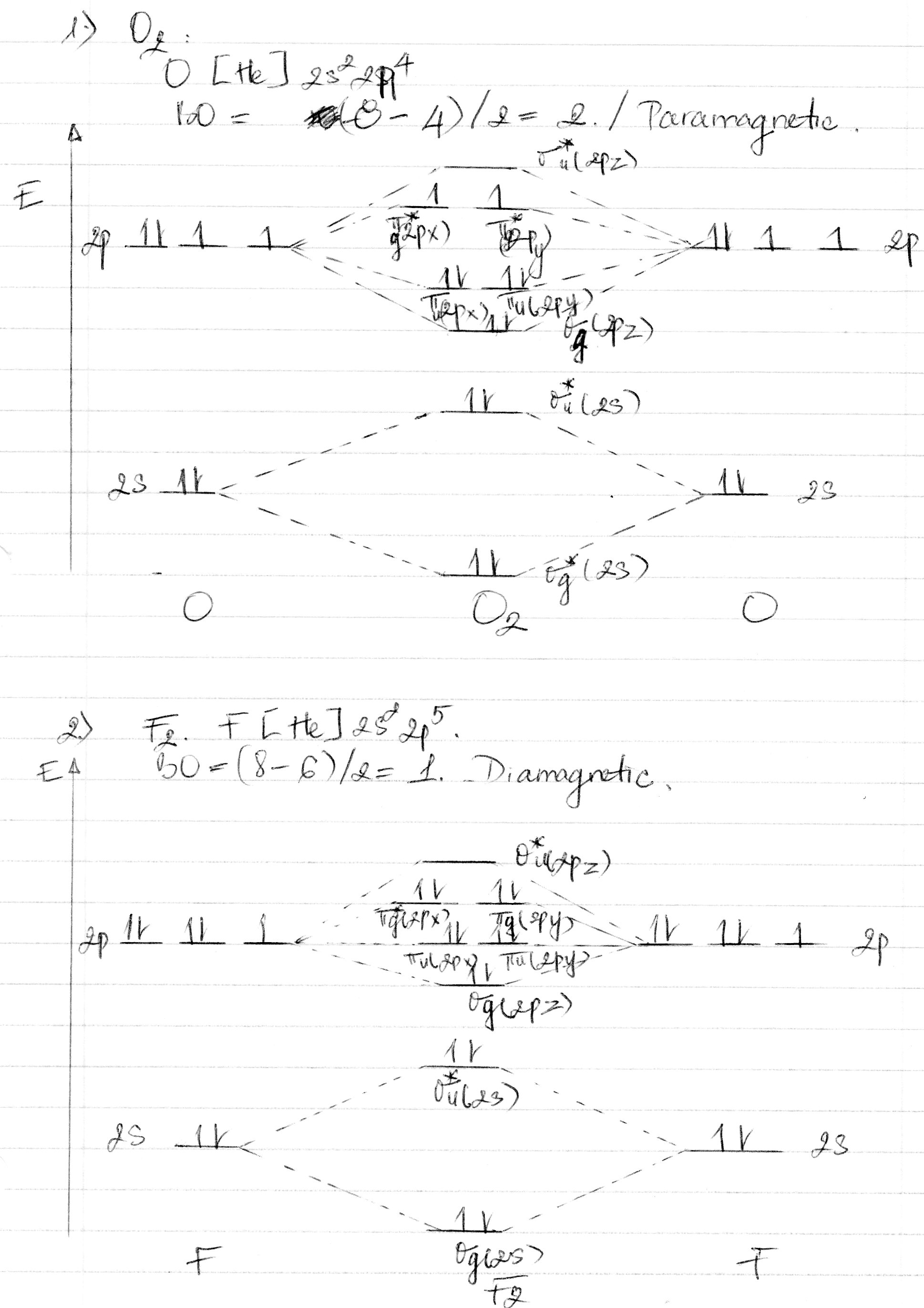
C- has a bond order of 2. C has bonding pairs and antibonding pair so it has a bond order of 2. O has bonding pairs and antibonding pairs so it has a bond order of 2. Excited state bond order = 3. Ground state bond order = 2. To get a negative charge on O you would have to have a single bond , so bond order is 1. Cis unstable diatomic carbon and if it exists, it. The antibonding orbital is empty. However, when you draw the Lewis structure of B you get a triple bond. I always thought bond order corresponded to the number of bonds. The higher the bond order , the stronger the chemical bond.
There are six bonding electrons and two antibonding electrons. Cdoes not follow the VSEPR. Write the electron configuration of Cmolecule. From the above electron configuration, but the values in the formula.
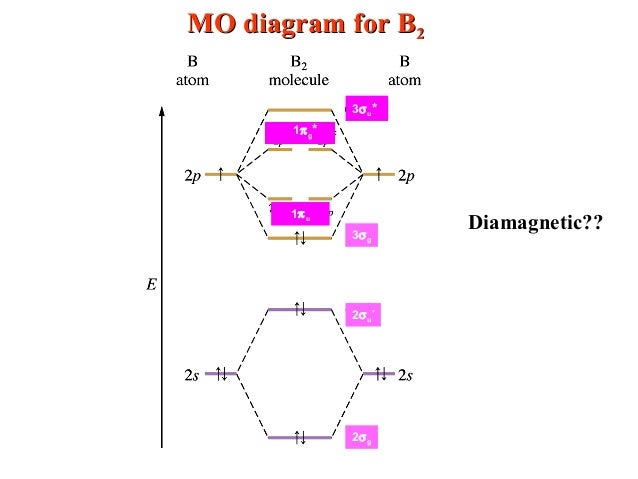
Chas a bond order of i. This is quantified by quantum mechanical, theoretical studies that show the bond orders to be ∼1. Antibonding orbitals are denoted by an asterisk symbol next to the associated type of molecular orbital.