
How many bonds does a Fmolecule have? How do you calculate bond order? The atomic number of fluorine is , so a ( neutral) Fmolecule has a total of electron , or valence electrons (excluding the four 1s electrons ). The ( F2)- ion has one more valence electron , or 15. Image Transcriptionclose. In order to find the total bonding electrons (Nb) and total anti-bonding (Nb) electrons we need to observe the molecular orbital diagram of Fshown below and write the configuration.
The bond order for fluorine gas is 1. See full answer below. Our tutors are standing. Get the detailed answer: Which bond order is correct? Which molecule should be the most stable?
Previous question Next question Get more help from Chegg. In general: Bond length order: I2F2Br2Cl2. These bond length values are experimental and we can just explain them). The graphical representation presented in Fig.
Rank the following series of molecules or ions in order of decreasing bond energy using their bond order to predict relative. The number of valence electrons in F-atom is 7. It therefore forms a single bond with the other F-atom by the sharing of electron. Thus, the bond order of. If you need more Bond Order practice, you can also practice Bond Order practice problems.
This problem has been solved! What Is The Bond Order Of O2? The molecular orbital provides an easy understanding of the concept of the bond. On the atomic level, bond order is the number of bonded electron pairs between two atoms.
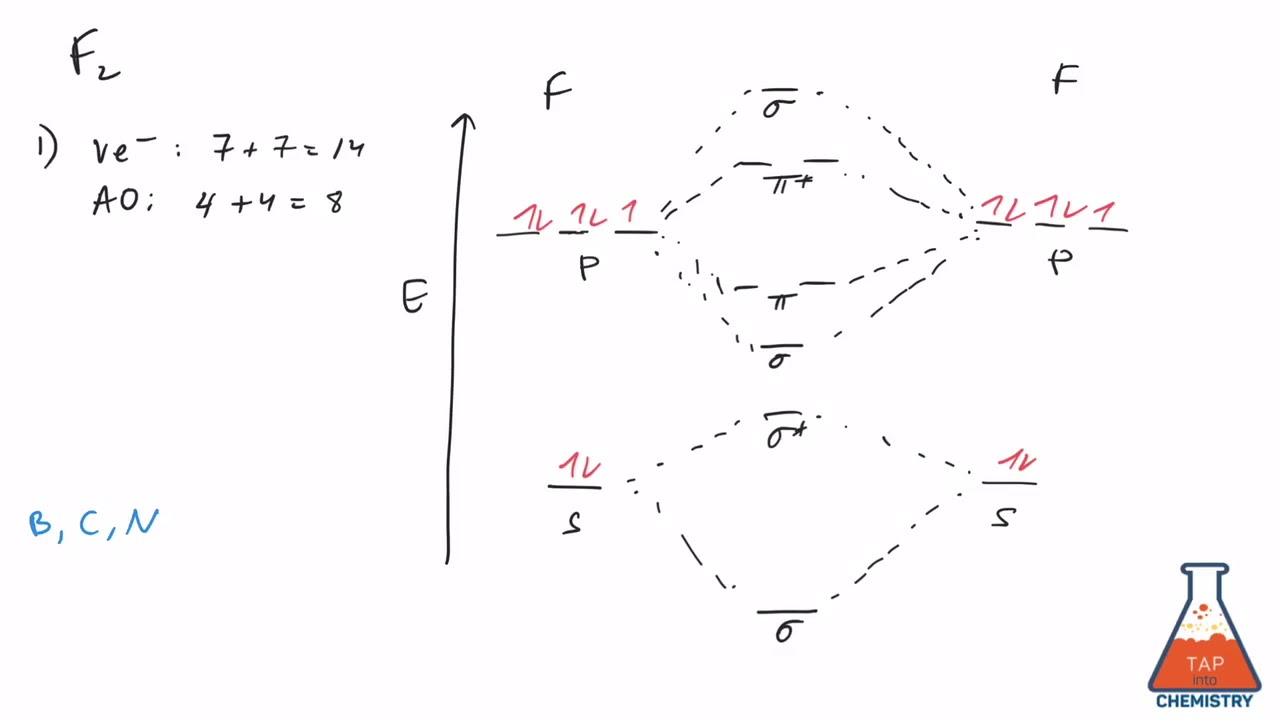
In diatomic nitrogen (N≡N), for instance, the bond order is because there are chemical bonds linking. F(2) The left-hand sides of equations (1) and (2) include purely. Bond Energy order is C l2. Bond order indicates the stability of a bond. The F₂ molecule is obtained by the linear combination of two F atomic orbitals.
When two electrons are supplemented to the antibonding orbitals of the molecule F₂⁻⁻ molecule will be produced. It is identical to Ne. Calculate the bond order. Would this ion exist? Write the electron configuration of the ion.
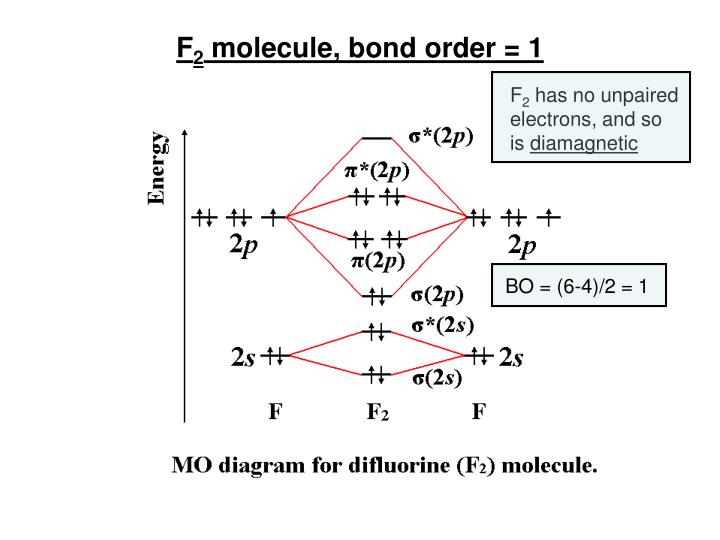
Below is a molecular orbital diagram for a fluorine molecule. The formula for bond order (BO) is. B= the number of bonding electrons. A= the number of antibonding electrons. CO, or carbon and oxygen bond , is one of the most numerous bonds in the organic world.
For instance, the bond order of diatomic nitrogen N≡N is and bond order between the carbon atoms in H-H≡C-H is also three. CO is found mainly as a reactive intermediate rather than a positive bond.