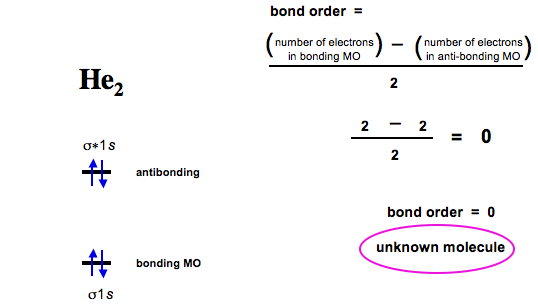
People exposed to beryllium compounds are at increased risk of developing beryllium sensitization and chronic beryllium disease (CBD). A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. I understand that Be cannot exist, since it has as many electrons in the antibonding as in the bonding orbitals. When the two beryllium 2s orbitals interact they split in two energy levels – one bonding (lower energy) and one antibonding (higher energy) – and when the four electrons of both Beryllium atoms are divided over these two new molecular orbitals (symmetric and antisymmetric, or g and u combinations of the 1s orbitals),both levels get fully occupied and there is no net energy gain and therefore no bond. Computational Chemistry in Drug Design While the descriptions of bonding described in this chapter involve many theoretical concepts, they also have many practical, real-world applications.
For example, drug design is an important field that uses our understanding of chemical bonding to develop pharmaceuticals. From the electronic configuration it is clear that there is no singly filled atomic orbital present in beryllium. Without the half filled orbital,the overlapping is not possible,therefore Bemolecule does not exist.
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. Calculate the bond order. Would this ion exist? Each Beryllium has electrons, and two orbitals.
Combining two Beryllium atoms would result in a total. Heteronuclear case (e.g., HCl) – Polar bonds. Here we introduce an electronegativity difference between the two atoms making the chemical bond. The energy of an electron in the H 1s orbital is higher (it is easier to ionize) than the electron in the chlorine 3p z orbital.
The molecular orbitals are filled in the same manner as atomic orbitals, using the Aufbau principle and Hund’s rule. The ground states of the above molecules are examined mainly through multi reference configuration interaction methods using an aug-cc-pVQZ basis set. This textbook survival guide was created for the textbook: Chemistry , edition: 11.

Learn to draw molecular orbital electron configuration energy diagrams. Figure “Molecular orbital. Double-ζ and triple-ζ basis sets containing both (multiple) polarization and diffuse functions were applied.
The gas-phase ion hydrates were produced by electrospray, and the hydration equilibria were determined in a reaction chamber attached to a mass spectrometer. On the atomic level, bond order is the number of bonded electron pairs between two atoms. In diatomic nitrogen (N≡N), for instance, the bond order is because there are chemical bonds linking.

Molar mass of Be(SO4)is 210. Convert between Be(SO4)weight and moles. Org online education free homework help chemistry problems questions. Week – Molecular Geometries. Wing area: 3sq ft (3 m 2) Empty weight: 3lb (6kg) Gross weight: 3lb (0kg) Powerplant: × RAF 1a V-air-cooled piston engine, hp (kW) Propellers: 4-bladed wooden fixed-pitch propeller.
If Be receives electrons, you get Be2. Now the anion has electrons in the inner layer and valence electrons in the second layer. Bonding Of Diatomic Molecules B BEChemistry. Mailstop: T- BE-03.
Department of Biological Sciences and Department of Chemistry , Biochemistry and Physics. It is used as an indicator of the stability of a chemical bond. Usually, the higher the bond order, the stronger the chemical bond. Most of the time, bond order is equal to the number of bonds between two atoms.
Orbital hybridization is fundamental to understanding organic chemistry. This is summed up in the following diagram, where a positive phase 2s orbital and a 2p orbital interact to produce an sp hybrid orbital. The plus charge removes one. This is considering all electrons, not just the valence electrons.
Whatever source which is giving you the 7-electron MO Diagram must be considering both valence and non-valence electrons (includes the 1s electrons). Chemistry Video Lessons Exam Reviews ACS Video Solutions Solutions Library Homework Help Organic Chemistry Video Lessons Exam. Simple cubic, body-centered cubic and face-centered cubic unit cells. General Chemistry II Study Sheets. Any time two electrons share the same orbital, their spin quantum numbers have to be different.
Whenever two electrons are paired together in an orbital, or their total spin is they are diamagnetic electrons. Arrange the following gaseous ions in order of increasing atomic radius. Which of the following methods is best for sepa-rating a one liter sample of two miscible liquids with a boiling point difference.