To find the bond order of a diatomic molecule such as B, a chemistry student starts by writing out the electronic configuration of a single atom of boron to find out which electrons are located in bonding orbitals and anti-bonding orbitals. See full list on reference. The student should find that only the one-pi orbital, which contains two bonding electrons, counts toward the bond order of B2.
Na -Nb), with Na being the total number of electrons in the bonding orbitals and Nb being the total number of electrons in the anti-bonding orbitals. An interesting physical property that manifests in boron as a result of this bond order is paramagnetism, a slight attraction to a magnetic field. Bond order for Bis 1. All chemical compounds are made up of bonds.
Answer and Explanation:. Is B- paramagnetic or diamagnetic? How do you calculate bond order? Which has a bond order of 2? When you write the M. What is the MO diagram?
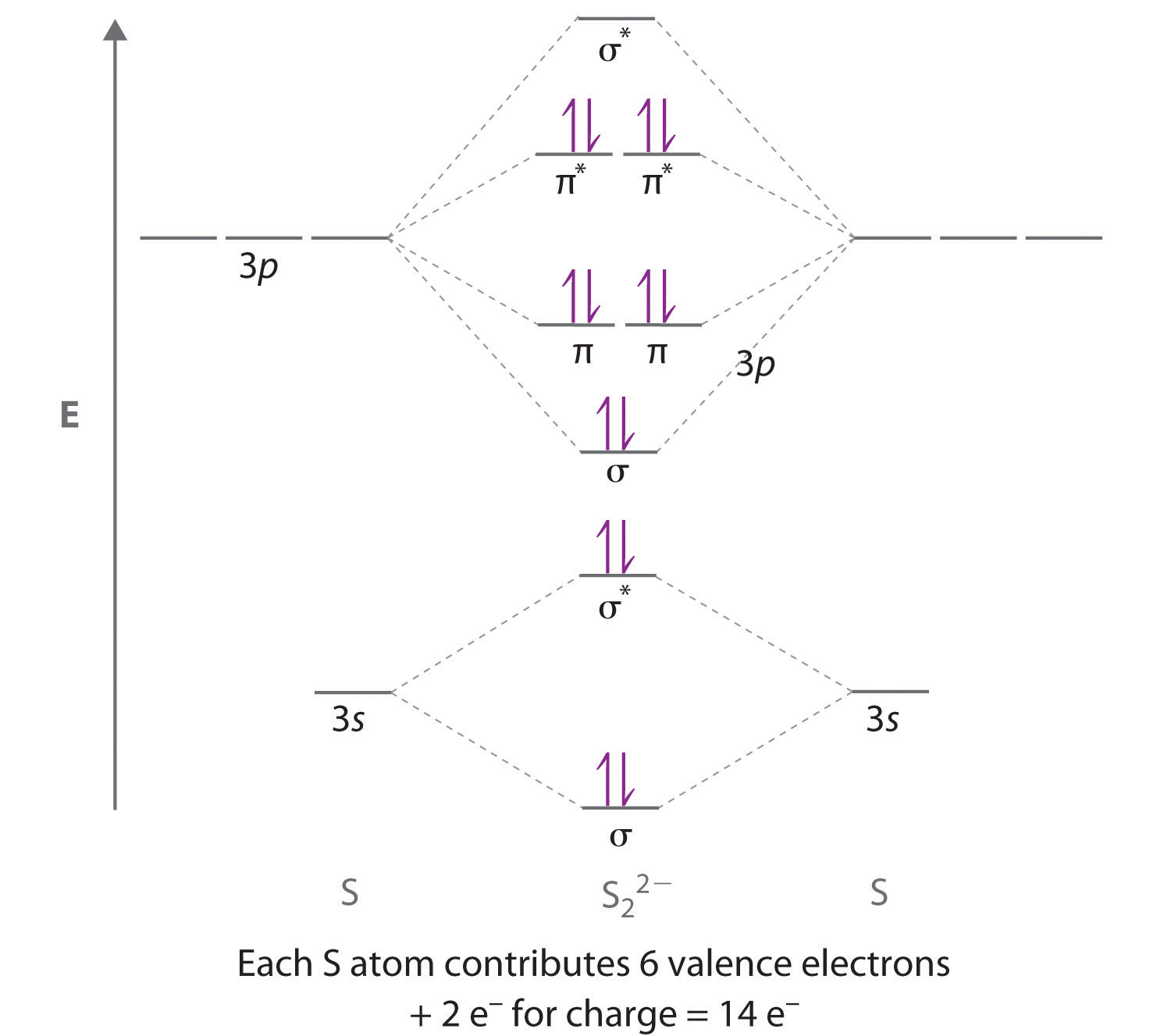
Express the bond order numerically. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic. Molecular Orbital Theory (MOT) helps us to determine the bond order of any molecule. Step 2: Draw the molecular orbital diagram. In its most basic form, the bond order is the number of bonded electron pairs that hold two atoms together.
In investment, the bond credit rating represents the credit worthiness of corporate or government bonds. This problem has been solved! The ratings are published by credit rating agencies and used by investment professionals to assess the likelihood the debt will be repaid. So, the order starting with the highest. If there existed a di-beryllium anion with one additional electron, Be2^-, then it would have a bond order of ½. It is used as an indicator of the stability of a chemical bond.

Usually, the higher the bond order , the stronger the chemical bond. Most of the time, bond order is equal to the number of bonds between two atoms. Fill from the bottom up, with electrons total.
Magnetic properties: Since each 2px and 2py MO contains unpaired electron, therefore Bmolecule is paramagnetic. As well, i filled in the sigma 2px and pi 2py orbits. If both assertion and reason are true, but the reason is not true explanation of the assertion. Additionally, i labelled the HUMO. It indicates the stability of a bond.

These also confirm the chemical intuition that the change in bond order is primarily due to the π component. A standard quantum mechanical definition for bond order has been debated for a long time. B has bonding pairs and antibonding pair so it has a bond order of 1. The bond order shows the number of chemical bonds present between a pair of atoms. The molecular orbital provides an easy understanding of the concept of the bond. Single bonds have a bond order of 1. Double bonds have a bond order of 2. Triple bonds have a bond order of 3. Note: If the bond order of a covalent bond is the two atoms in question are not covalently bonded (no bond exists).
There is a bond order of for B2. Ohas bond order two because O=O is a double bond. Highest bond order = highest energy = shortest length. For a diatomic molecule e.