How does plasma form? What are plasma particles? The other fundamental states of matter are liquids, solids, and gases.
Typically, plasma is made by heating a gas until its electrons have sufficient energy to escape the hold of the positively charged nuclei. As molecular bonds break and atomsgain or lose electrons, ions form. Just like your parents put fuel into their car, energy gets added to a gas,.
Atoms or molecules can acquire a positive or negative electrical charge when they gain or lose. An inductively coupled plasma is a plasma that is energized (ionized) by inductively heating the gas with an electromagnetic coil, and contains a sufficient concentration of ions and electrons to make the gas electrically conductive. It is sometimes referred to as the fourth state of matter, distinct from the soli liqui and gaseous states. Called also blood plasma. From Longman Dictionary of Contemporary English.
When separated from the rest of the bloo plasma is a light yellow liquid. The main role of plasma is to take nutrients, hormones, and proteins to the parts of the body that need it. Cells also put their waste products into the plasma. The plasma then helps remove this waste from the body. It also contains salts and enzymes.
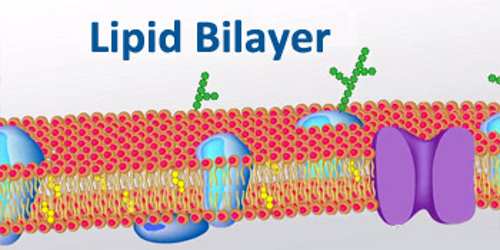
And it has antibodies that help fight infection, plus proteins called albumin and fibrinogen. It is also simply called the cell membrane. The main function of the plasma membrane is to protect the cell from its surrounding environment.
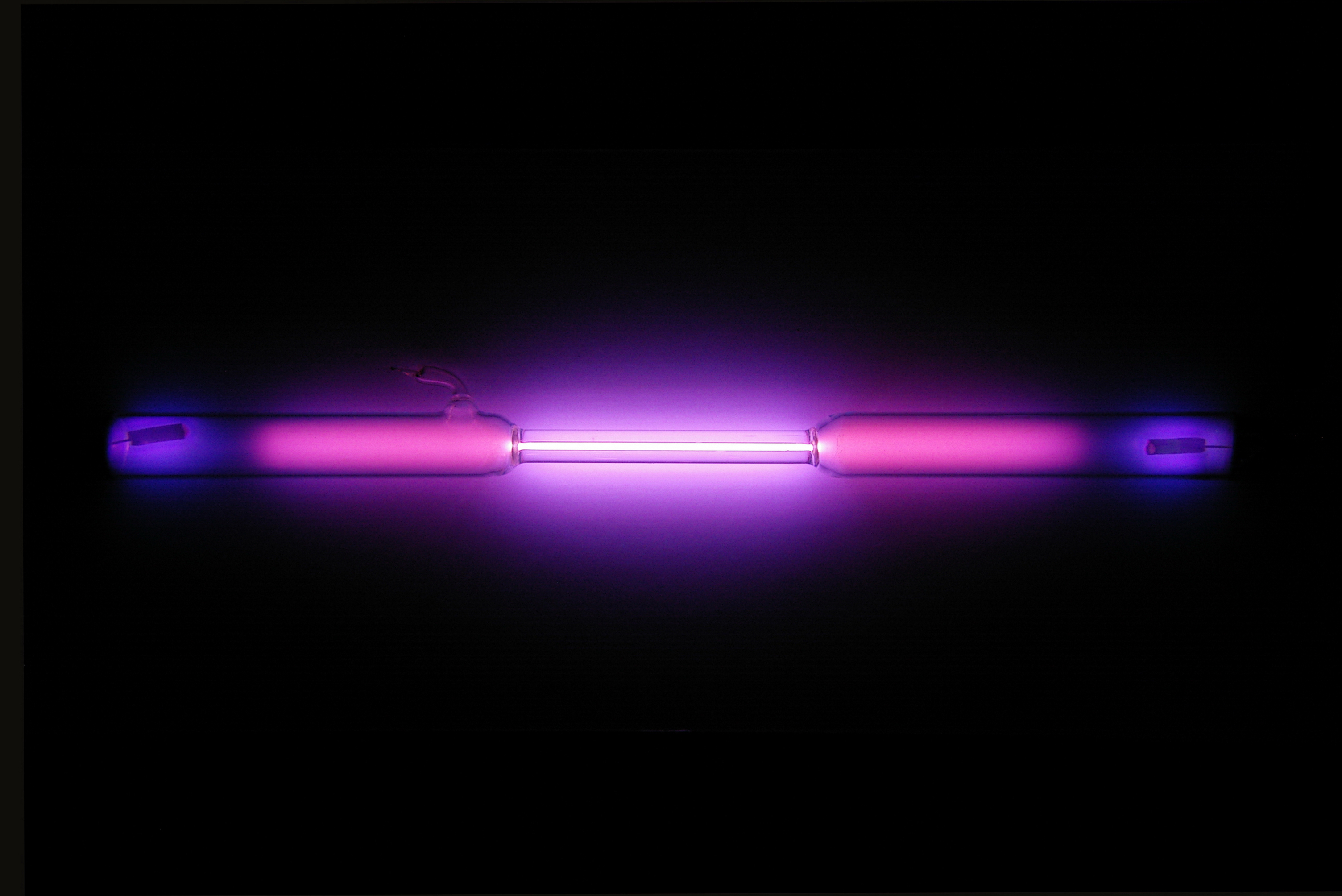
Plasma carries water, salts and enzymes. A physical state of matter which exists at extremely high temperatures in which all molecules are dissociated and most atoms are ionized. A plasma is a gas that has been energized to the point that some of the electrons break free from, but travel with, their nucleus. Gases can become plasmas in several ways, but all include pumping the gas with energy.
A spark in a gas will create a plasma. This is called ionization. It in negatively charged electrons , and positively charged ions. It can expand to fill a container. However, the particles in plasma are ionized (carry an electric charge) and very widely separated from each other.

A plasma does not necessarily involve the process of combustion, burning or oxidation of material. The argument that the term flame can only be applied to a process of combustion, burning or oxidation is dependent on the definition of flame. Normally, the electrons in a soli liqui or gaseous sample of matter stay with the same atomic nucleus. Ordinary electric current is the flow of electrons through a wire conductor (see electricity). Ionized in this case means that at least one electron has been dissociated from a. Other components include dissolved proteins (e.g. serum albumins, globulins, fibrinogen), glucose, clotting factors, electrolytes, hormones, carbon dioxide, and oxygen.
It consists chiefly of water (about by volume). In humans, plasma serves as a medium to transportessential substances in blood. These components remain when the cells’ components are taken out.
Serum is the liquid component of the blood that remains once the blood has clotted. On the other han plasma is the liquid part that remains when clotting does not take place and the anticoagulant is added. Furthermore, the plasma accounts for a higher percentage of the total volume while serum accounts for a small percentage of the total blood volume.