What are the paramagnetic elements? Is Lidiatomic or paramagnetic? Bromine is paramagnetic. This is because the 4p subshell has a value of 4p5. The unpaired electron gives it.
Ferromagnetism is a large effect, often greater than that of the applied magnetic fiel that persists even in the absence of an applied magnetic field. And From the MOT concept Bedoesn’t exists as its Border is and in case of para or dia it is diamagnetic. A paramagnetic electron is an unpaired electron.
An atom is considered paramagnetic if even one orbital has a net spin. An atom could have ten diamagnetic electrons, but as long as it also has one paramagnetic electron, it is still considered a paramagnetic atom. The other two are diamagnetic. So we have these two definitions.
Diamagnetic atoms repel magnetic fields. Paramagnetic and diamagnetic. And we can figure out if atoms or ions are paramagnetic or diamagnetic by writing electron configurations.
Hence beis neither diamagnetic nor paramagnetic as it does not exist. Is B2- paramagnetic or diamagnetic ? Bond order: Molecular Orbital Theory (MOT) helps us to determine the bond order of any molecule. Still have questions? From the molecular orbital diagram of N predict its bond order and whether it is diamagnetic or paramagnetic.
Question: What is the bond order of B-? Similar is the behaviour of steel, cobalt, and nickel. Such substances are called ferromagnetic substances. Some substances are only weakly attracted by a magnet, while some are repelled by it.
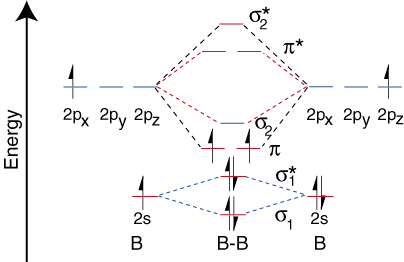
They are called paramagnetic and diamagnetic respectively. It is diamagnetic due to the absence of any unpaired electron. Bmolecule: The electronic configuration of B atom (Z = 5) is. B molecule is formed by the overlap of atomic orbitals of both boron atoms.
A number of valence electrons of each boron atom = 3. ProbleWhat is the bond order of B2-? B is paramagnetic because it has two unpaired electrons, one in each of its p orbitals. C is diamagnetic because all of its electrons are paired. NO has an odd number of electrons an therefore, must be paramagnetic. I read something recently that said BN has been observed in the gas phase, is paramagnetic , and has a vibrational frequency lower than N2.
These properties can be explained by the molecular orbital diagram of BN. How can i find para or diamagnetic for. Such materials or substances are called diamagnetic.
This chemistry video tutorial focuses on paramagnetism and diamagnetism. It shows you how to identify if an element is paramagnetic or diamagnetic by writing. What problems can arise in using nondisclosure and noncompete agreements to protect intellectual property. Click here to buy a book, photographic periodic table poster, card deck, or 3D print based on the images you see here! Concept introduction: The strong-field ligands lead to pairing of electrons and lead to diamagnetic species, while the low-field ligand do not have tendency to pair up the electrons and therefore forms.
In this example, the ions contain only paired electrons, and the atoms contain only unpaired electrons.